In the article previous to this, Part I, we discussed design and statistical issues related to clinical trial designs for COVID-19 research. As part of the discussion about clinical trial designs comes along interpretation of results. As clinical trials for COVID-19 vaccine trials have continued into Phase III, eventually early promising results were released about efficacy about certain vaccines. This information was released to the general public but this also brought about a lot of confusion.
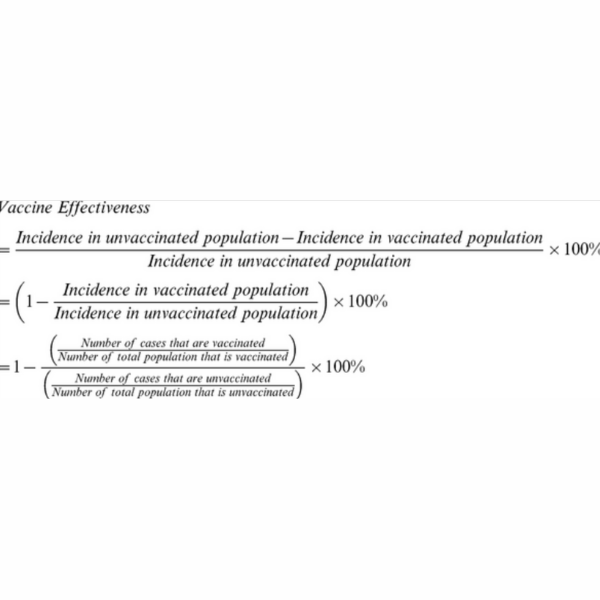
The general public simply was unable to grasp the results of the efficacy or effectiveness of the vaccine. They did not understand the calculations for such results or how they were derived. Suddenly the public was expected to all be epidemiologists and be fully comfortable with such language. The percent efficacy rates were calculated from relative risks that were ascertained during the trials. These were calculated from number infected in the treatment group compared to number infected in the placebo group. This ratio of number of percent infected in both groups was calculated (or the relative risk of infection in the vaccinated group divided by the relative risk of infection in the unvaccinated group) and then 1 minus that rate was calculated as the % vaccine efficacy. Most people in the public were unaware of relative risks. Explaining the calculation in simple terms has helped to some extent, but ultimately, it was the interpretation of the efficacy that has been the most confusing. For example what exactly did 90% efficacy mean? Did this mean that with the vaccine, there was only a 10% chance of not getting COVID-19? Unfortunately this explanation is a commonly thought one about the efficacy.
This is where we circle back to the relative risk. If people understood the calculation was based on the relative risk then they might have better appreciation for the risk interpretation. A 90% efficacy rate for a given vaccine means that on average a person has a 90% reduced risk of being infected by COVID-19; it doesn’t mean they have a 90% coverage of not being infected by COVID-19 or any other interpretation as such. Honestly, it is very confusing for all, most especially a nervous general public. Being able to explain the epidemiology and statistics to the everyone is something we as statisticians should actively embrace.
Written by
Usha Govindarajulu
In the article previous to this, Part I, we discussed design and statistical issues related to clinical trial designs for COVID-19 research. As part of the discussion about clinical trial designs comes along interpretation of results. As clinical trials for COVID-19 vaccine trials have continued into Phase III, eventually early promising results were released about efficacy about certain vaccines. This information was released to the general public but this also brought about a lot of confusion.
The general public simply was unable to grasp the results of the efficacy or effectiveness of the vaccine. They did not understand the calculations for such results or how they were derived. Suddenly the public was expected to all be epidemiologists and be fully comfortable with such language. The percent efficacy rates were calculated from relative risks that were ascertained during the trials. These were calculated from number infected in the treatment group compared to number infected in the placebo group. This ratio of number of percent infected in both groups was calculated (or the relative risk of infection in the vaccinated group divided by the relative risk of infection in the unvaccinated group) and then 1 minus that rate was calculated as the % vaccine efficacy. Most people in the public were unaware of relative risks. Explaining the calculation in simple terms has helped to some extent, but ultimately, it was the interpretation of the efficacy that has been the most confusing. For example what exactly did 90% efficacy mean? Did this mean that with the vaccine, there was only a 10% chance of not getting COVID-19? Unfortunately this explanation is a commonly thought one about the efficacy.
This is where we circle back to the relative risk. If people understood the calculation was based on the relative risk then they might have better appreciation for the risk interpretation. A 90% efficacy rate for a given vaccine means that on average a person has a 90% reduced risk of being infected by COVID-19; it doesn’t mean they have a 90% coverage of not being infected by COVID-19 or any other interpretation as such. Honestly, it is very confusing for all, most especially a nervous general public. Being able to explain the epidemiology and statistics to the everyone is something we as statisticians should actively embrace.
Written by
Usha Govindarajulu
https://journals.plos.org/plosone/article/file?type=thumbnail&id=info:doi/10.1371/journal.pone.0041785.e001