September 14, 2022
According to a recent article in Nature Communications, regular rapid testing provided two-fold benefits: identifying infectious individuals and providing positive tests sufficiently early during infection that treatment with antivirals can effectively inhibit development of severe disease. They focused specifically on the extent of nirmatrelvir-associated treatment benefits that are accrued among high-risk populations when rapid tests are administered at various intervals.
Their analysis required two primary sources of data to estimate probability-weighted risk ratios (RRs): positivity curves and treatment efficacy-associated RRs. They go on to describe how to parameterize the test positive probabilities, they used an estimated lateral flow test “(LFT)-like” positivity curve generated from a Hellewell et al. Bayesian model, which reports the posterior probability of testing positive for each day since infection from 0 to 30 days, across 4000 Markov chain Monte Carlo (MCMC) draws. That is, while the patient population in this analysis were routinely administered PCR – and not antigen – tests, the authors provided two scenarios estimating expected test positive probabilities under antigen testing, by defining lower CT thresholds that would better reflect the diagnostic ability of LFTs. To parameterize relative risks (RRs) of hospitalization, they used data reported in the latest EPIC-HR press release, namely the number of hospitalized subjects in the treatment and placebo groups, and the total size of each treatment arm, stratified by when treatment was initiated.
To estimate RRs over a range of days since symptom onset, they then fit a log-binomial model to the reported hospitalization counts by assigning a series of treatment-labeled 1 s and 0 s to reflect the recorded hospitalizations and non-hospitalizations within each arm, with covariates representing days since symptom onset and treatment group. They assumed a linear relationship between the continuous predictors (day and the treatment x day interaction) and the log risk of hospitalization as well as an additive effect of treatment. Then from their model fit, they extracted RR estimates for each day on the range [0,7] days since symptom onset and defined a RR of 1 (i.e., no treatment effect) thereafter. They additionally fit a logistic model to determine whether their results were sensitive to whether RRs and ORs were employed, and observed nearly equivalent results, which as they claim they expected due to the rare outcome of hospitalization.
They then employed another method again from Hellewell et al to estimate probabilities of pre-symptom onset symptomatic detection where they assumed a CT threshold of 28. They then used the probability of either first testing positive at day of testing to weight among the treated or testing negative at all days, weighted by a RR of 1. They then averaged these weighted RRs over all possible testing sequences and they replicated this process over the 4000 simulations of positivity data and for each testing strategy (every other day, every 3 days, once a week, and once every 2 weeks). To summarize results for each strategy, they extracted median weighted RRs and an accompanying 95% confidence interval (CI). To match hospitalization risks reported with the time reference of days since symptom onset to test positive probabilities reported with the time reference of days since infection, they sampled incubation periods (the times from infection to symptom onset) – assuming a lognormal distribution consistent with pooled estimates from a McAloon et al. meta-analysis at each iteration and used the rounded incubation periods to index time relative to symptom onset rather than infection and identify the appropriate RR for each day. They allowed for possible delays in x days from testing positive to treatment by additionally setting a variable which shifts this period by x days. In our main analyses, we assumed a zero-day positive-test-to-treatment delay corresponding to potential plans to distribute antiviral pills to households to store for immediate use if necessary and further assumed full treatment coverage.
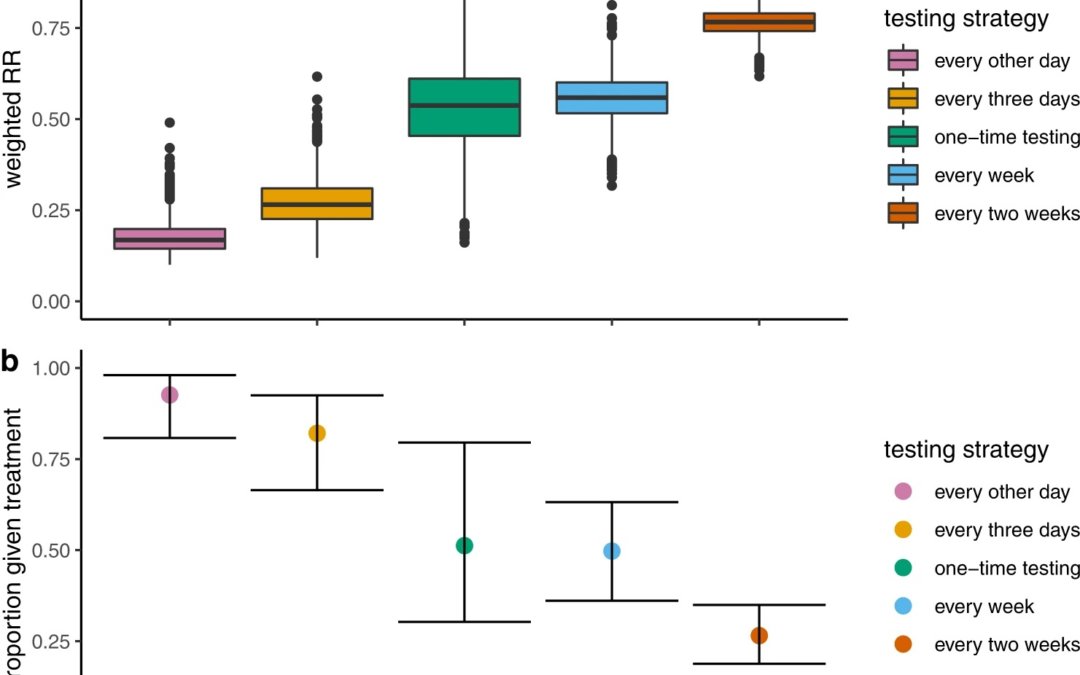
Their long list of methods and lengthy description and assumption on the part of the reader’s knowledge about Hellewell et al’s simulation methods and McAloon et al’s meta-analysis methods to the following results. They found that when strategies were used to administer tests more often that this was associated with greater reductions in the risk of hospitalization, with weighted risk ratios for testing every other day to once every 2 weeks ranging from 0.17 (95% CI: 0.11–0.28) to 0.77 (95% CI: 0.69–0.83) and correspondingly, higher proportions of the infected population benefiting from treatment, ranging from 0.26 (95% CI: 0.18–0.34) to 0.92 (95% CI: 0.80–0.98), respectively. As they pointed out, reduced treatment delays coupled with increased test and treatment coverage have a critical influence on average treatment benefits, confirming the significance of access.
Written by,
Usha Govindarajulu
Keywords: COVID-19, rapid tests, antiviral treatments, Sars-CoV-2
References
Menkir, T.F., Donnelly, C.A. The impact of repeated rapid test strategies on the effectiveness of at-home antiviral treatments for SARS-CoV-2. Nat Commun 13, 5283 (2022). https://doi.org/10.1038/s41467-022-32640-2
Hellewell J, et al. Estimating the effectiveness of routine asymptomatic PCR testing at different frequencies for the detection of SARS-CoV-2 infections [Internet]. Epidemiology. 2020.
McAloon, C. et al. Incubation period of COVID-19: a rapid systematic review and meta-analysis of observational research. BMJ Open 10, e039652 (2020).
https://media.springernature.com/full/springer-static/image/art%3A10.1038%2Fs41467-022-32640-2/MediaObjects/41467_2022_32640_Fig1_HTML.png?as=webp