
by Usha Govindarajulu | Feb 17, 2022 | Biostatistics, Blog, COVID-19, Usha Govindarajulu
February 22, 2022 In a pre-print article published recently, the authors used life table and survival analyses methods to compare probability of dying from COVID-19 against probability of dying from other causes using data from the province Hubei of China, of which...
by Usha Govindarajulu | Feb 4, 2022 | Biostatistics, Blog, COVID-19, New York, Professor, Usha Govindarajulu
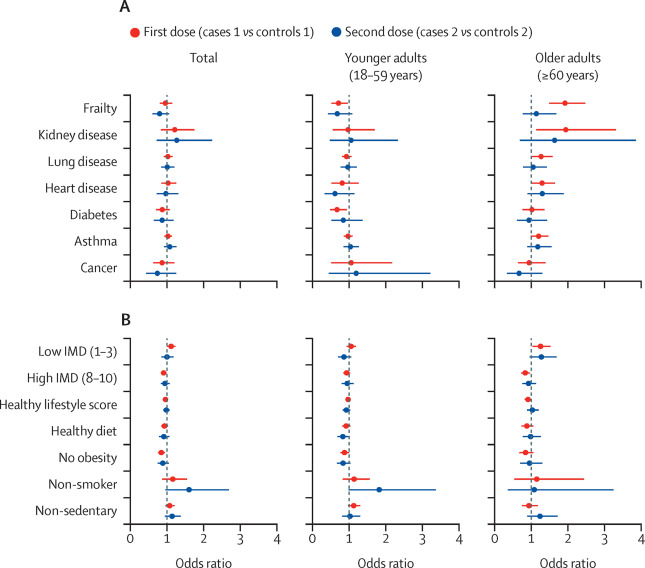
February 2, 2022 A recent article in the Lancet Infectious Diseases presents a large scale study of post-vaccination infection conducted in the United Kingdom to assess potential long-haul symptoms. It was a prospective, community-based, nested, case-control study...

by Usha Govindarajulu | Jan 20, 2022 | Biostatistics, Blog, COVID-19, Professor, Usha Govindarajulu
January 18, 2022 A recent article in the New England Journal of Medicine (NEJM) presents results from a large cohort study from New York State on COVID-19 vaccine effectiveness. They used data for 8,690,825 adults in New York State to assess the effectiveness of the...
by Usha Govindarajulu | Jan 7, 2022 | Biostatistics, Blog, COVID-19, Healtcare
January 5, 2022 With the ongoing rise in COVID-19 cases due to the Omicron variant of Sars-Cov-2, we searched for some actual studies on the change in vaccine efficacy due to Omicron exposure. In one pre-print article, Buchan et al studied 3,442...
by Usha Govindarajulu | Dec 22, 2021 | Biostatistics, Blog, COVID-19, Usha Govindarajulu
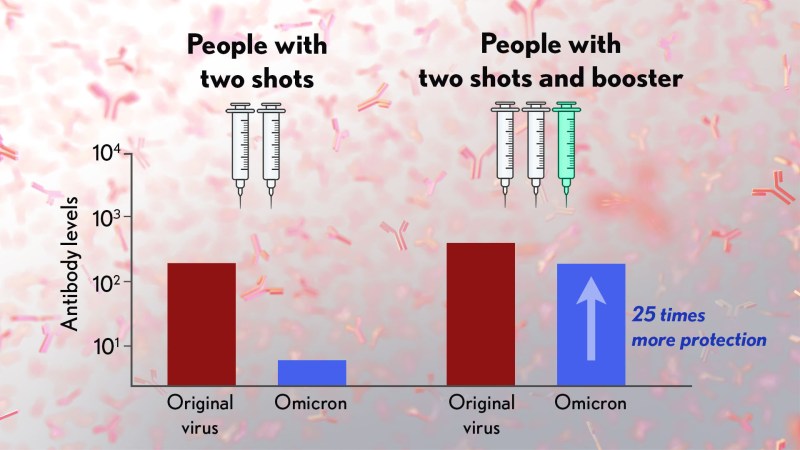
The new variant of COVID-19, Omicron, and boosters from vaccines has been receiving a lot of press. In an article published in BMJ on December 14, 2021, “Covid-19: Omicron and the need for boosters”, the authors take us through a detailed summary about how a booster...





