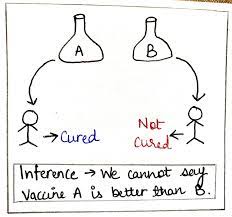
P-values and vaccine efficacy
As the companies continue to re-evaluate vaccine efficacy against new variants, have they discussed p-values? Are they analyzing re-updated data again and again and correcting for inflation of the Type I error rate?? Given the p-value controversy in statistics and the controversy of vaccine efficacy, statisticians have to wonder if these topics have been considered together. Even if this topic is being considered, it is hardly discussed. One can only imagine it must have been very difficult to plan these trials and have any plan for conserving the Type I error rate. It would seem to be hard to design any sequential probability or alpha spending for these studies that were run for vaccines against COVID-19 because the designers may not have any idea how to plan for interim looks at the data because how would they know how many looks they would need at the data? Certainly, this topic has not been discussed in the literature or brought out as any concern in the main news since there are so many other pressing issues behind the vaccine developments. As statisticians, we have our concerns about the data, like how it is being handled and analyzed and this is one of those major topics. While this has a lot of control in most regular clinical trials, has not been accompanied in the literature of these COVID-19 vaccine trials. However it would be an important point to know about the handling of Type I error in these vaccine trials and the challenges behind them. We hope to learn more.
Written by: Usha Govindarajulu