April 23, 2025
The authors have discussed a sample size calculation for restricted mean survival time (RMST) in augmented tests. The RMST was developed as an alternative measure of survival that is non-parametric and does not reply on parametric constraints. It had been further extended to include a truncation time of when to truncate the study time and also a difference and ratio of RMSTs between two groups comparison was developed for use in comparing survival differences between randomized trials. Their primary focus involved the augmented tests based on RMST and development of sample size calculations for these augmented tests. The tests relied on reference data, which includes data from past clinical trials, and target study, which is what is being designed.
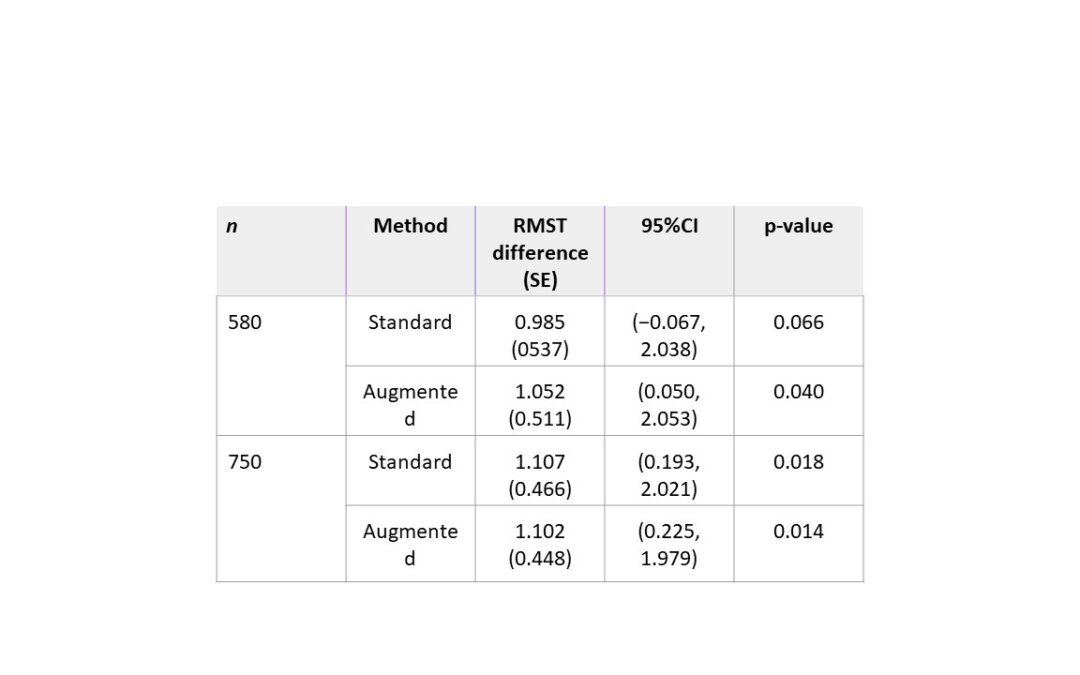
They derived an augmented test for the RMST difference. They showed AUG in their Equation 4 so assuming this is short for augmented. They then derived power formulas for the treatment difference which they called theta. In regards to a randomized clinical trial, if one has prior data on the control group then one can calculate the power with their formula by estimating the baseline survival function and the censoring survival function with the Kaplan-Meier method. However one must note that these quantities are influential on the calculation of the predicted power for the standard and augmented tests. Also, the predicted power of the augmented tests depends on the variance-covariance matrix of the covariates and the martingale residuals so therefore the prior study estimates need to be accurate to power for the target study.
They ran their method through simulations and real dataset analyses. In general the method was designed to be used when the sample size used is not too small. In the first real dataset example, the reference data was not close to the target study. In the second example they had better success with their augmented test. Overall, one has to be careful with what information is used from the reference data for the power calculation.
Written by,
Usha Govindarajulu
Keywords: survival analysis, sample size, RMST, randomized clinical trials
References:
Hattori S and Uno H (2025) “On Sample Size Determination for Augmented Tests Base on Restricted Mean Survival Time in Randomized Clinical Trials” Biometrical Journal, https://doi.org/10.1002/bimj.70046