by Usha Govindarajulu | Mar 27, 2025 | Biostatistics, Blog, Usha Govindarajulu
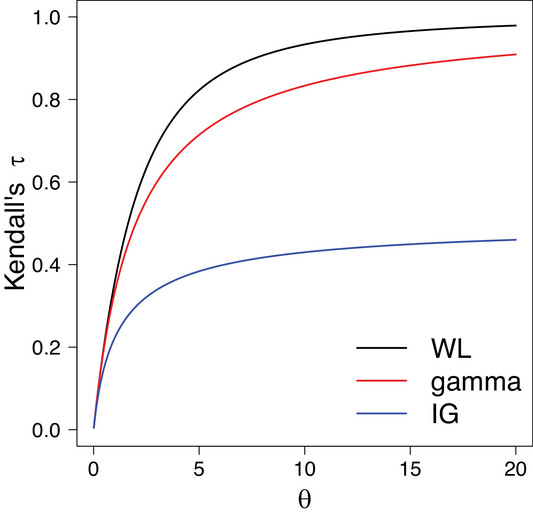
March 26, 2025 Their primary goal of their paper was to introduce a novel frailty model based on the weighted Lindley (WL) distribution for modeling clustered survival data. They used the weighted Lindley as the frailty distribution. This was a two parameter...
by Usha Govindarajulu | Feb 26, 2025 | Biostatistics, Blog, Usha Govindarajulu
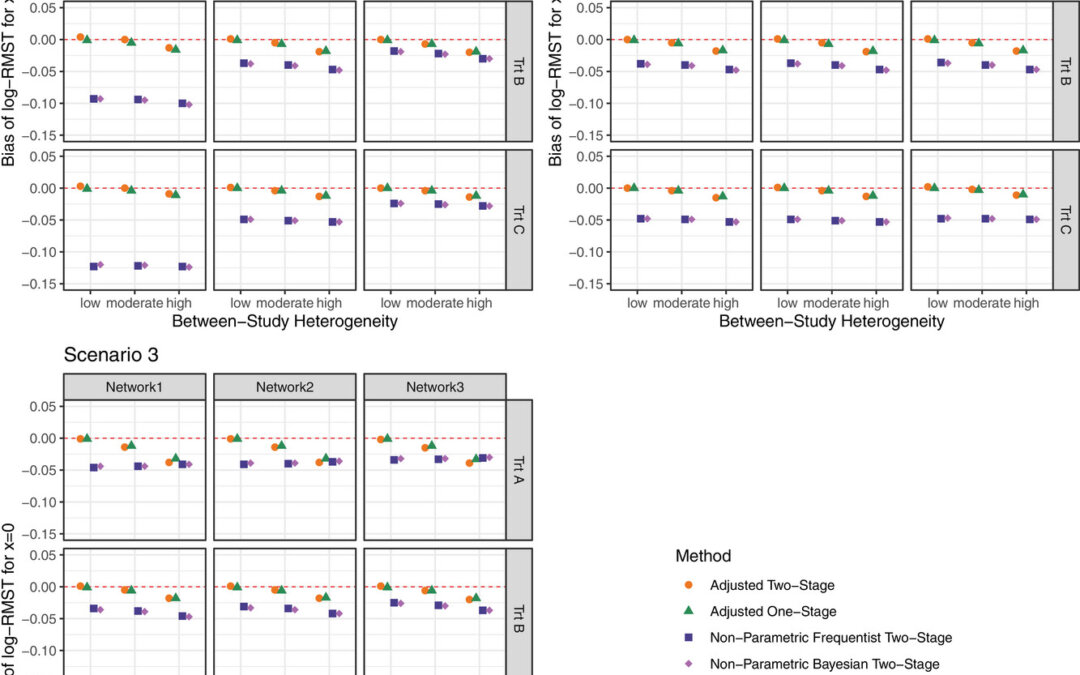
February 26, 2025 The authors have used network meta-analysis (NMA) to compare multiple treatments simultaneously using restricted mean survival time (RMST) with individual level data (IPD). Since the NMA was mainly used with aggregate data, they had to extend this...
by Usha Govindarajulu | Feb 12, 2025 | Biostatistics, Blog, Usha Govindarajulu
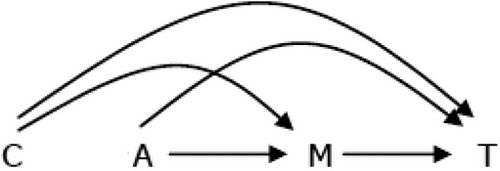
February 12, 2025 The authors focused on causal mediation analysis in survival analysis, more specifically with additive hazards modeling with an exposure by mediator interaction term. The causal mediation framework had come about over time from the mediation area and...
by Usha Govindarajulu | Jan 29, 2025 | Biostatistics, Blog, Usha Govindarajulu
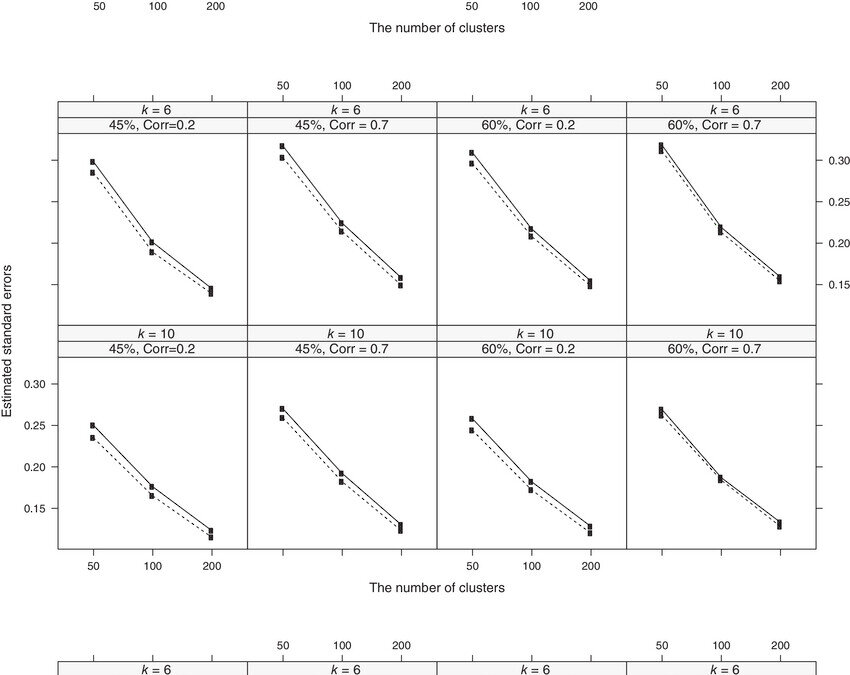
January 29, 2025 Many analyses have used clustered data but not everyone considers that clusters may be informative. For example, generalized estimating equations (GEE) and marginal proportional hazards model have been widely used to estimate population-average...
by Usha Govindarajulu | Jan 15, 2025 | Biostatistics, Blog, Healtcare, Usha Govindarajulu
January 15, 2025 In this paper the authors proposed prognostic accuracy measures for recurrent event data. They did so by developing semiparametric estimators for making inferences about the prognostic accuracy measures that accommodate recurrent events. Usually a...

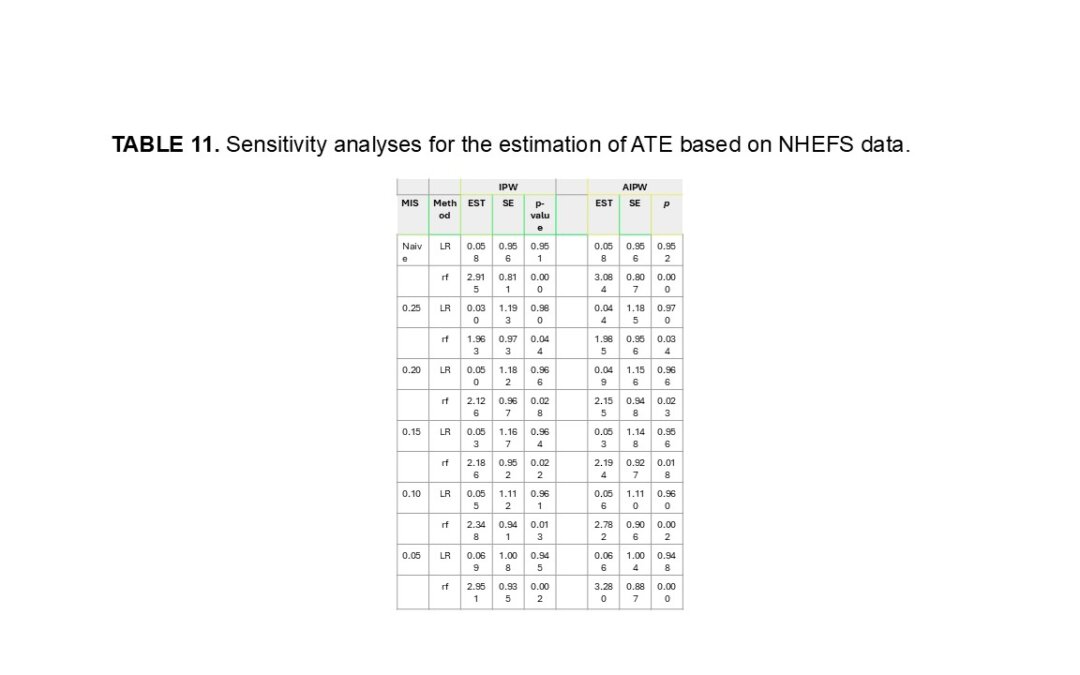
by Usha Govindarajulu | Jan 2, 2025 | Biostatistics, Blog, Usha Govindarajulu
January 1, 2024 The authors were interested in the average treatment effect (ATE) which reflects how the treatment affects the potential outcome. In order to estimate ATE, propensity scores have been adapted for their estimation. such as the inverse probability...






